
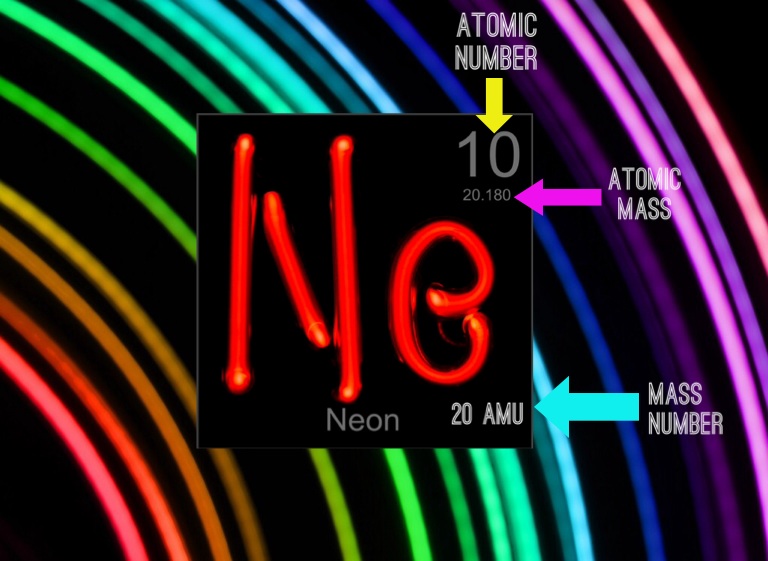
The next, after krypton had been removed, was a gas which gave a brilliant red light under spectroscopic discharge. The gases nitrogen, oxygen, and argon had been identified, but the remaining gases were isolated in roughly their order of abundance, in a six-week period beginning at the end of May 1898. Neon was discovered when Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. Neon was discovered in 1898 by the British chemists Sir William Ramsay (1852–1916) and Morris Travers (1872–1961) in London. Neon gas-discharge lamps forming the symbol for neon Since air is the only source, it is considerably more expensive than helium. It is commercially extracted by the fractional distillation of liquid air. Neon is used in some plasma tube and refrigerant applications but has few other commercial uses. The red emission line from neon also causes the well known red light of helium–neon lasers. Neon gives a distinct reddish-orange glow when used in low- voltage neon glow lamps, high-voltage discharge tubes and neon advertising signs. Even the outer atmosphere of Jupiter is somewhat depleted of neon, although for a different reason. As a result, it escaped from the planetesimals under the warmth of the newly ignited Sun in the early Solar System. The reason for neon's relative scarcity on Earth and the inner (terrestrial) planets is that neon is highly volatile and forms no compounds to fix it to solids. It composes about 18.2 ppm of air by volume (this is about the same as the molecular or mole fraction) and a smaller fraction in Earth's crust. Although neon is a very common element in the universe and solar system (it is fifth in cosmic abundance after hydrogen, helium, oxygen and carbon), it is rare on Earth. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates.ĭuring cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Neon is chemically inert, and no uncharged neon compounds are known. The name neon is derived from the Greek word, νέον, neuter singular form of νέος ( neos), meaning 'new'. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. Liquid neon is now commercially available and is finding important application as an economical cryogenic refrigerant.Neon is a chemical element with the symbol Ne and atomic number 10. Neon and helium are used in making gas lasers.
#NEON NE TV#
UsesĪlthough neon advertising signs account for the bulk of its use, neon also functions in high-voltage indicators, lightning arrestors, wave meter tubes, and TV tubes. Of all the rare gases, the discharge of neon is the most intense at ordinary voltages and currents. It is compact, inert, and is less expensive than helium when it meets refrigeration requirements. It has over 40 times more refrigerating capacity per unit volume than liquid helium and more than three times that of liquid hydrogen. In a vacuum discharge tube, neon glows reddish orange.

The ions, Ne+, (NeAr)+, (NeH)+, and (HeNe+) are known from optical and mass spectrometric studies. It is still questionable if true compounds of neon exist, but evidence is mounting in favor of their existence. Neon is a very inert element, however, it has been reported to form a compound with fluorine. Natural neon is a mixture of three isotopes. It is obtained by liquefaction of air and separated from the other gases by fractional distillation. Neon is a rare gaseous element present in the atmosphere to the extent of 1 part in 65,000 of air. Discovered by Ramsay and Travers in 1898.


 0 kommentar(er)
0 kommentar(er)
